
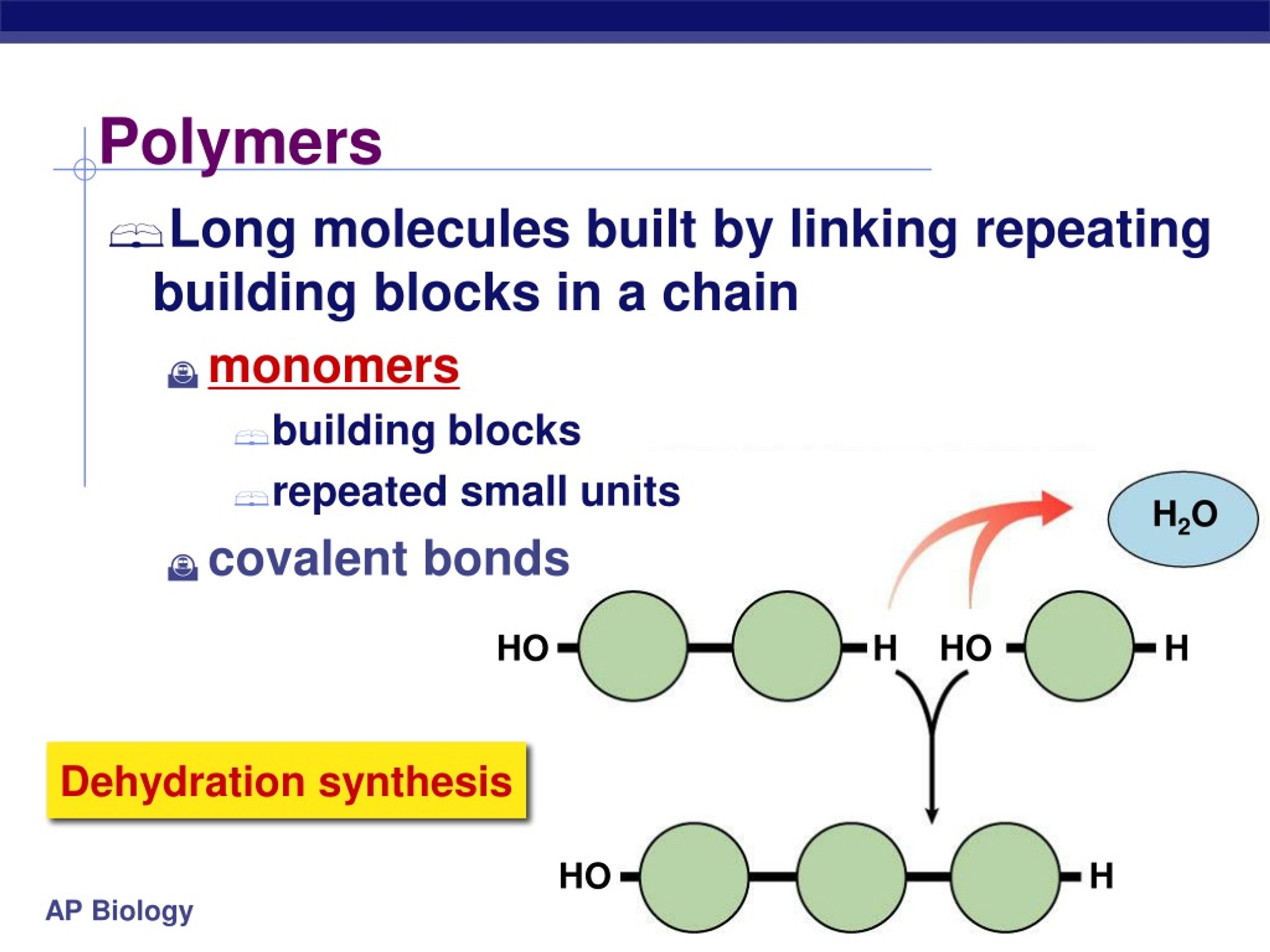
Living creatures on Earth use only ‘left-handed’ amino acids. Keeping the analogy with your hands, the two enantiomers of an amino acid are often referred to as being ‘right-’ and ‘left-handed’ enantiomers of the same molecule.įigure 3: Amino acids come in right and left handed versions. The situation is somewhat similar to your two hands, which have the same ‘formula’ (Palm 1Fingers 4Thumb 1), but no amount of rotating of either hand can get the two to perfectly superpose. Because of the arrangement of chemical groups around the ‘central’ carbon atom of an amino acid, it is possible to make two versions (called enantiomers) of an amino acid that have identical chemical formulas, but that are geometrically different, each being the mirror image of the other. One of the peculiar properties of amino acids is that they are “chiral” or “handed” molecules (Figure 3). For example, over 70 different amino acids have been identified in meteorites. The chemical group or side chain at position “R” can be almost anything, but life on Earth uses slightly over 20 different amino acids. All amino acids have a similar basic structure like that in Figure 1, with different amino acids differing only in the specific structure that lies in the chemical group or side carbon chain labeled “R”.įigure 1: The basic structure of all amino acids.Īs two examples, here are schematics of the simple amino acids glycine (R = H, a hydrogen atom) and alanine (R = CH 3, a methyl group, Figure 2).įigure 2: The structures of the simple amino acids glycine (left) and alanine (right). Knowing this, we can deduce the correct answer to this question.Amino Acids and Their Production during the Photolysis of Astrophysically Relevant IcesĪmino acids are important biological molecules that serve as the basic molecular building blocks of proteins (including enzymes) used by all living things on Earth. Makes it easier to store as it does not interfere with the balance of water in They can then convert glucose into a large, insoluble polymer called starch, which Many plants produce their own sugars in the form of glucose through the process of Glucose is a small, simple sugar molecule. So, these cannot be the monomer subunits of starch either. Cellulose and glycogen are also carbohydrate polymers made of many repeating units of Starch is a large carbohydrate made of many repeating units of a much smallerĪmino acids are the monomers of proteins, not carbohydrates. Means many, referring to the fact that polymers are made up of many repeating The word “monomer” contains the word mono- which means one, while the prefix poly. Starch is a polymer made up of many monomers. A large molecule, starch, is formed from multiple smaller molecules. This question presents us with an example of an anabolic reaction. The formation of these bonds requires an input of energy, which is supplied by In contrast, anabolic reactions form bonds between small molecules, joining them Stored in cells in the high-energy bonds of a molecule called ATP so it can beĮasily accessed when needed. The breaking of bonds in catabolic processes releases energy, which is temporarily You might have learned that metabolic reactions can be grouped into those that areĬatabolic reactions are those that break the bonds in large, complex molecules,īreaking these molecules down into smaller subunits. Metabolism describes all of the chemical reactions that occur within living organisms This question asks us about a metabolic reaction, in which smaller molecules are Which monomer subunits join together to form starch in a metabolic reaction? (A) Glucose, (B) glycogen, (C) cellulose, or (D) amino acids. In some metabolic reactions, large molecules are made from smaller ones, as shown in


 0 kommentar(er)
0 kommentar(er)
